A 40 Year Old Diabetic Teacher Having Chest Pains Continuing for 45 Minutes
A 63-year-old man is referred to your clinic for evaluation of chest pain. He reports several weeks of exertional chest tightness associated with mild shortness of breath when he is mowing the lawn. These symptoms never occur at rest. He has a history of type 2 diabetes and hypertension.
He has no significant family history. He has a ten pack year smoking history and quit smoking twenty-five years prior. His medications include metformin 1000 mg twice daily, lisinopril 10 mg daily, aspirin 81 mg daily, and hydrochlorothiazide 25 mg daily.
His blood pressure is 139/87 mm Hg with a pulse of 75 beats per minute (bpm) and a body mass index (BMI) of 31. His exam is notable for slightly diminished dorsalis pedis and posterior tibial pulses with normal lower extremity perfusion and no edema. Exam was otherwise unremarkable.
His electrocardiogram (ECG) shows normal sinus rhythm at 83 bpm, and criteria for left ventricular hypertrophy with nonspecific ST and T-wave changes.
His laboratory values are significant for HbA1c 8.4%, total cholesterol 227 mg/dL, high-density lipoprotein cholesterol (HDL-C) 37 mg/dL, triglycerides 255 mg/dL, low-density lipoprotein cholesterol (LDL-C) 142 mg/dL, TSH of 1.3 mIU/L and serum creatinine of 1.1 mg/dL.
| Figure 1 | |
 | |
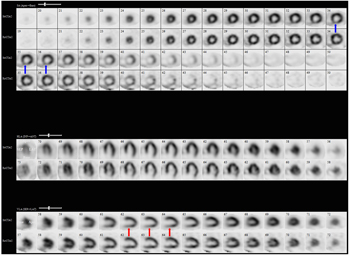
Given his symptoms, coronary disease risk factors and ECG changes, he undergoes a one-day exercise Tc-99m myocardial perfusion study. He exercises a total of seven minutes, 49 seconds on a standard Bruce protocol and achieves 9.8 METS. Peak heart rate is 135 bpm (86% of his maximum predicted heart rate). He develops mild chest pain with peak exercise, and the test is terminated due to leg fatigue. At peak stress, his ECG exhibits 1 mm horizontal ST depressions in the inferior leads that resolve two minutes into recovery. Perfusion images are shown (Figure 1).
The correct answer is: D. Add a statin, a beta-blocker, and a long-acting nitrate.
Diabetes mellitus is a disease with an increasing worldwide prevalence and significant implications for cardiovascular health. From 1976-2001, patients with diabetes enrolled in the Framingham Heart Study had nearly a threefold increased risk of cardiovascular disease mortality compared to those without diabetes.1 Additionally, studies have found up to a 45% incidence of myocardial infarction (MI) in diabetic patients over a seven year period.2 Derangements in nitric oxide bioavailability, endothelial cell function, vascular smooth muscle function, and thrombosis have all been implicated in diabetic vascular disease.3
| Figure 2: Tc 99m myocardial perfusion imaging shows a reversible perfusion defect in the mid and basal inferior walls (red arrow) and the basal inferoseptal wall (blue arrow). | |
 | |
The patient in this case exhibits poor glycemic control with a HbA1c above target (<7%), which has additional implications for his cardiovascular risk. Among patients with type 2 diabetes, the relative risk for fatal coronary heart disease is 1.16 for each one-percentage point increase in HbA1c.4 Additionally, his age, gender, elevated total cholesterol, low HDL-C, and hypertension further increase his risk of significant cardiovascular disease.5 This patient, like many patients with diabetes and insulin resistance, has a lipid profile characterized by low HDL-C, high triglycerides, and a modest increase in LDL-C, which are predominantly small dense particles. The overall particle number is increased as would be reflected in an elevated apolipoprotein B (apoB) level. These small, dense LDL particles are more easily oxidized, enter the arterial wall more readily, are cleared more slowly, and are thus considered more atherogenic. His physical exam is suggestive of peripheral arterial disease (PAD), and coincident coronary artery disease is found in up to 90% of patients with definitive PAD.6 Aggressive treatment of these cardiovascular risk factors can improve both morbidity and mortality. In the Steno-2 trial, 160 patients with type 2 diabetes were randomized to either intensive therapy of conventional therapy.7 Targets for the intensive therapy group included HbA1c <6.5%, fasting total cholesterol <175 mg/dL, fasting triglycerides <150 mg/dL, systolic blood pressure <130 mm Hg, and diastolic blood pressure <80 mm Hg. After a mean of 13.3 years of follow up, intensive therapy was associated with a decrease risk of cardiovascular death (hazard ratio 0.43) and cardiovascular events (hazard ratio 0.41). Therefore, our patient warrants intensive lipid, blood pressure, and glycemic control independent of his angina and stress test results.
Our patient's testing is suggestive of flow-limiting coronary artery disease (CAD) in what is likely a right coronary artery distribution. His symptoms are consistent with stable angina rather than an acute coronary syndrome. Several studies have examined the utility of revascularization in addition to optimal medical therapy (OMT) in patients with stable angina. In A Very Early Rehabilitation Trial (AVERT), patients with CAD (defined as 50% or greater stenosis of one or more coronary arteries) and an LDL of 115 mg/dL or greater were randomized to either percutaneous coronary intervention (PCI) or aggressive lipid lowering with high-dose atorvastatin.8 The study population was 84% male and 95% Caucasian, and 78% of those enrolled experienced angina at baseline. After 18 months of follow up, the atorvastatin group exhibited a 36% decrease in ischemic events. The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) study group investigated the role of PCI in addition to OMT in patients with type 2 diabetes and stable ischemic heart disease. Patient with left main disease were excluded from the trial. In this study population, PCI provided no improvement in five-year survival or the rate of major cardiovascular events.9 Similarly, the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial found that PCI in addition to OMT did not significantly decrease the rate of major cardiovascular events when compared to medical therapy alone in patients with stable CAD.10 Again patients with severe left main stenosis were excluded from the study. Per the 2012 ACC/AHA guidelines on stable ischemic heart disease, diabetic patients with stable angina should receive medical therapy, including aspirin, beta-blocker, moderate-to-high-intensity statin, and either a long-acting nitrate or calcium channel blocker.11 Furthermore, our patient's stress test is highly suggestive of single vessel disease without left main or proximal left anterior descending (LAD) artery involvement. Therefore, at this point in the clinical scenario, option A is incorrect, and option D is the next most appropriate step.
If symptoms persist despite medical therapy, it is reasonable to pursue revascularization. In patients with three-vessel CAD or left main disease, PCI has an increased rate of major adverse cardiac and cerebrovascular events compared to CABG.12 The Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) Trial compared revascularization with coronary artery bypass graft (CABG) versus PCI in patients with diabetes and severe CAD. In this population, the majority of whom had multivessel CAD, CABG resulted in a significant decreased rate of major adverse cardiac or cerebrovascular events after one year (12.4 vs. 17.8%).13 Patients also report improvements in angina frequency, physical limitations, and quality of life with CABG compared to PCI.14,15
Cardiac rehabilitation plays an important role in secondary prevention as well as ongoing risk factor modification. In fact, data show that rates of death and MI decrease with increased attendance at rehabilitation sessions.16 Stable angina, as in this patient, is an appropriate indication for referral to cardiac rehabilitation, but he should first receive maximal medical therapy to treat his anginal symptoms prior to referral. Therefore, option B is incorrect.
Option C is incorrect. While an assessment of left ventricular function can be an important part of this patient's management, there were no signs or symptoms suggestive of congestive heart failure on exam. Therefore, an echocardiogram should not be the first step in his management.
Clinicians are increasingly using coronary computed tomography angiography (CCTA) to evaluate for the presence of CAD. In a study of 122 patients with diabetes, atherosclerotic plaques were identified in 95% of subjects using CCTA.17 Similarly, an analysis of 138 patients with type 2 diabetes undergoing CCTA found that 75.2% exhibited coronary disease in at least two vessels with LAD artery involvement in 35.9% of the study population.18 A meta-analysis of 9,592 patients found that obstructive CAD (>50% luminal stenosis) on CCTA had a positive likelihood ratio of 1.70 for major adverse cardiac events.19 Conversely, the absence of obstructive CAD had a negative likelihood ratio of 0.008. In our patient, however, we have already found evidence of moderate ischemia on stress imaging. Per the 2010 American College of Cardiology/American Heart Association guidelines on CCT, subsequent CCTA after stress imaging showing this degree of ischemia is classified as an inappropriate use of the test.20 Therefore, option E is incorrect.
References
- Preis, SR, Hwant, S, Coady, S, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950-2005. Circulation 2009;119:1728-35.
- Haffner, SM, Lehto, S, Ronnemaa, T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:227-34.
- Creager, MA and Luscher, TF. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003;108:1527-32.
- Selvin, E, Marinopoulos, S, Berkenblit, G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421-31.
- Wilson, PWF, D'Agostino, RB, Levy, D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47.
- Golomb, BA, Dang, TT, and Criqui, MH. Peripheral arterial disease: morbidity and mortality implications. Circulation 2006;114:688-99.
- Gaede, P, Lund-Andersen, H, Parving, H, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580-91.
- Pitt, B, Waters, D, Brown, WV, et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. N Engl J Med 1999;341:70-6.
- The BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503-15.
- Boden, WE, O'Rourke, RA, Teo, KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503-16.
- Fihn, SD, Gardin, JM, Abrams, J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol 2012;60(24):e44-e164.
- Serruys, PW, Morice, M, Kappetein, AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72.
- Farkouh, ME, Domanski, M, Sleeper, LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375-84.
- Abdallah, MS, Wang, K, Magnuson, EA, et al. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA 2013;310,15:1581-90.
- Cohen, DJ, Van Hout, B, Serruys, PW, et al. Quality of life after PCI with drug-eluting stents or coronary-artery bypass surgery. N Engl J Med 2011;364:1016-26.
- Hammill, BG, Curtis, LH, Schulman, KA, et al. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly medicare beneficiaries. Circulation 2010;121:63-70.
- Gao, WG, Lu, B, Sun, ML, et al. Comparison of atherosclerotic plaque by computed tomography angiography in patients with and without diabetes mellitus and with known or suspected coronary artery disease. Am J Cariol 2011;108:809-13.
- Chu, Z, Yang, Z, Dong, Z, et al. Characteristics of coronary artery disease in symptomatic type 2 diabetes patients: evaluation with CT angiography. Cardiovasc Diabetol 2010;9:74-81.
- Hulten, EA, Carbonaro, S, Petrillo, SP, et al. Prognostic value of cardiac computed tomography angiography. J Am Coll Cardiol 2011;57:1237-47.
- Taylor, AJ, Cerqueira, M, Hodgson, JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. J Am Coll Cardiol 2010;57(22):1864-94.
Source: https://www.acc.org/education-and-meetings/patient-case-quizzes/a-63-year-old-man-with-diabetes-and-coronary-artery-disease
Postar um comentário for "A 40 Year Old Diabetic Teacher Having Chest Pains Continuing for 45 Minutes"